Cyanuric Acid
Is it possible to have too much of a good thing?
By Greg Schmidt
Cyanuric acid (CYA) is both a good and bad element in an outdoor pool. As an Aquatic Facility Operator course instructor and a pool operator for many years, I write this article to dispel some of the mystery around CYA and its reputation. Having just read a document by a respected source giving advice about CYA that is not consistent with the science presented here, I want to clear up any confusion about CYA that is still prevalent.
Photo: © Can Stock Photo / Gudella
First, let’s look at why it’s a good thing.
Hypochlorous acid (HOCl), or free chlorine, is subject to dissipation from UV rays in sunlight, which reduces residual potentially to levels below oxidation rates and even health-code minimums. HOCl is the form of chlorine that operators want in their pools. It sanitizes and oxidizes unwanted combined chlorine. Established standards for Oxidation Reduction Potential (ORP) in pools are 650 mV to kill bacteria and viruses, and 830 to 850 mV to provide excellent oxidation of combined chlorine. The goal of CYA use is to retain HOCl in adequate concentration to accomplish these two functions.
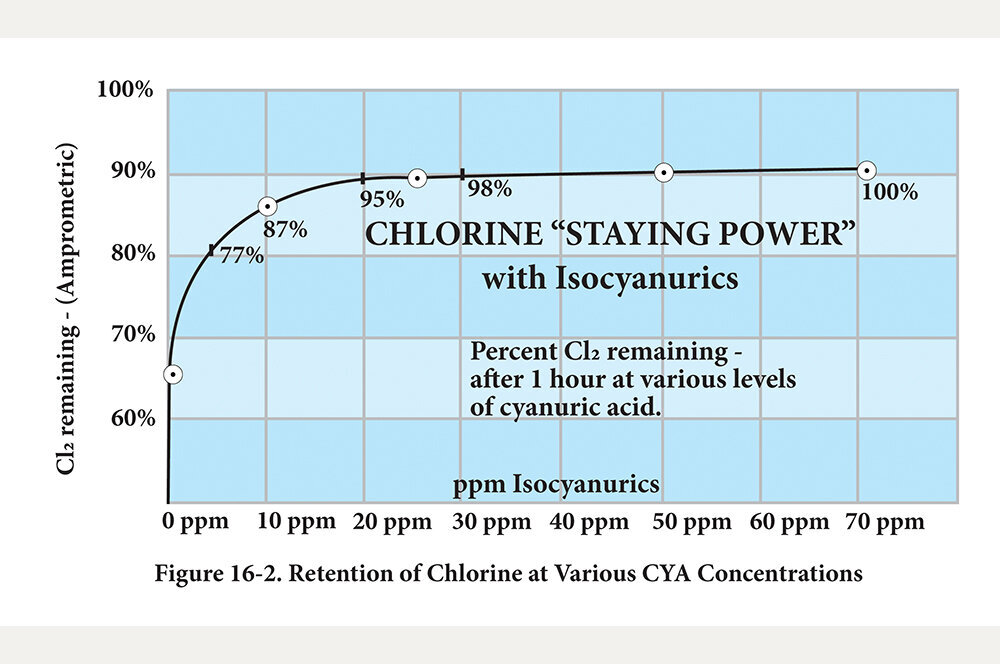
How does it work? CYA makes a loose chemical bond with HOCl to retain it and prevent sunlight dissipation. It’s called a “stabilizer” because this bond makes HOCl more stable, thus less likely to be lost to sunlight. It’s the instability of the HOCl molecule that makes it such a terrific sanitizer and oxidizer. As such, I strongly encourage operators of outdoor pools to use CYA at the level of 10 to 15 PPM to retain 87 to 90 percent HOCl. The absolute maximum level of CYA in a pool should be 20 PPM. For best results, start diluting water at 15 PPM to avoid ever getting to 20 PPM. Do not pay attention to the higher retention rates at higher CYA concentrations. At 20 PPM, there will be about 95-percent retention, but that’s where it has already become a bad thing.
Since it keeps the good chlorine in the water, why is it a bad thing?
At 95-percent retention, chlorine’s effectiveness at 20 PPM is down nearly 80 percent!
It becomes a bad thing when the concentration is too high because it stabilizes too much HOCl, which works best when unstable. The more HOCl that is stabilized, the less effective it is at oxidizing and sanitizing. When adding CYA, the drop in chlorine effectiveness is dramatic.
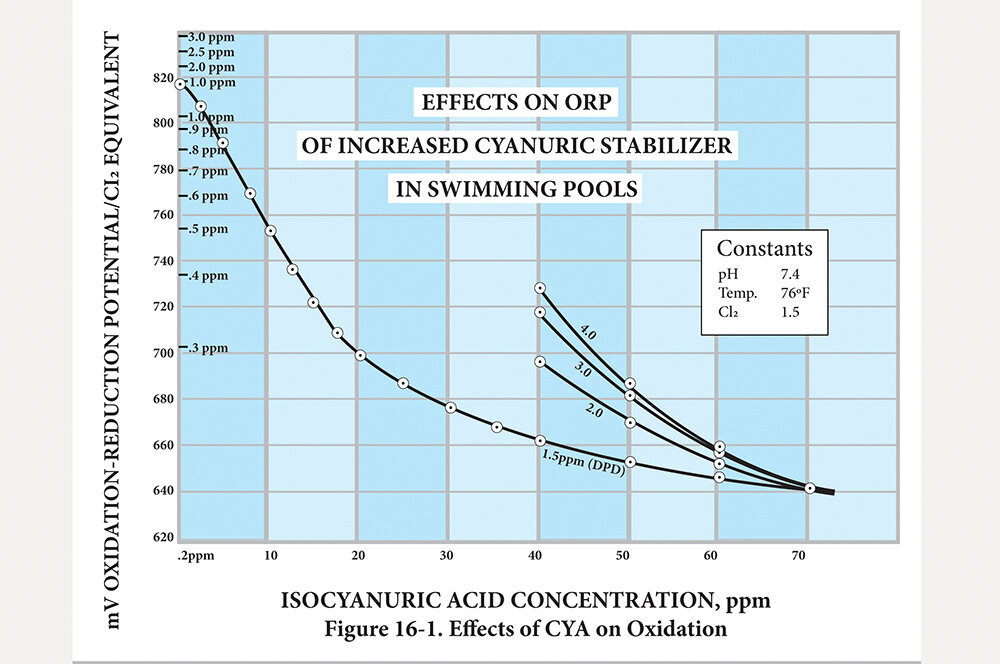
For example, at 4.0 PPM HOCl, pH of 7.4 and 40 PPM CYA, the ORP is only about 730 mV, which is not sufficient to eliminate combined chlorine or prevent its formation. With zero CYA, ORP should be about 840 to 850 mV at 4.0 PPM and a pH of 7.4—right at the target level to provide excellent oxidation.
Never shoot for “100-percent retention.” If you’re an operator who keeps the HOCl level at the minimum of 1.5 PPM, and the pH is 7.4, the addition of CYA is outright dangerous when attempting to achieve 100-percent retention. With zero CYA, ORP is about 820 mV. At 20 PPM CYA, it has already fallen to about 700 mV! At the 100-percent retention level of 70 PPM, ORP is under 650 mV and is not even killing the bacteria, let alone oxidizing any combined chlorine. Do you remember the statement about not paying attention to higher retention rates at higher CYA concentrations? This is why. See Figure 16-1.
This second chart demonstrates the effect of retention vs. CYA concentration. It speaks to the previous statements made above about why 10 to 15 PPM should be the limit and never allowing CYA to go above 20 PPM. One needs to really study both charts to fully understand the relationships between retention, concentration, and ORP.
The use of CYA is one of the three “detractors” of chlorine effectiveness, along with high combined chlorine and high pH. These three items all cause ORP to be compromised, and thus should be avoided. My mantra for terrific water quality is “it’s all about ORP!” CYA’s ideal concentration is zero because at zero PPM, none of the HOCl is too stable to be effective. The other two factors should also be as low as possible: pH at 7.3 and combined chlorine at zero. Combined chlorine has the same stabilizing effect of CYA. Like CYA, NH2Cl is far more stable than HOCl and is a lousy oxidizer and sanitizer.
What about Trichlor and Dichlor? They’re good, right?
I’m afraid not. Both of these chemicals are chlorine sources that are frequently sold by pool-supply companies to operators because they kill two birds with one stone, so to speak. They are chlorine and CYA in one. Although Trichlor may provide 90 percent available chlorine at a pH effect of around 3.0, it’s not as good as one might think.
The problem with both Trichlor and Dichlor is that CYA is embedded into the chemical, so there is no control over the amount of CYA buildup. I don’t recommend using either of these products in an outdoor pool and certainly not in a spa. That’s because of the buildup of CYA. It’s very hard to get rid of CYA once it’s in a pool. It tends to stick to everything, and even with a prudent dilution regimen, CYA will eventually build up on the heat exchanger, pipes, impellers, etc.
So what do I do?
Do use CYA in an outdoor pool to retain some of the HOCl, but not more than 15 PPM, and use dilution throughout the season to keep the level at 10 to 15 PPM. Do NOT exceed 20 PPM ever.
Do NOT use CYA in a hot tub.
Use calcium hypochlorite or sodium hypochlorite as a chlorine source.
Do keep the pH as low as possible within the local health code: 7.2 to 7.3. That will maximize ORP.
Do NOT use dichlor or trichlor in a pool or hot tub. It will erode control over CYA levels.
Buy pure CYA. It is an acid, with a pH effect of about 4.0. One pound of the granules will raise 120,000 gallons 1 PPM. Use proportions to determine how much to put into a pool. So, if a pool is 60,000 gallons, it will need 0.5 pounds for each PPM, and to get to the target of 10 PPM, it will need 10X 0.5 = 5.0 pounds.
0.5 lbs = x lbs = 5.0 pounds
1 PPM 10 PPM
Greg Schmidt, AFOI, LGIT/WSIT, is the Aquatic Center Manager for Eastern Washington University in Cheney, Wash. Reach him at (509) 359-4252, or leos@ewu.edu.